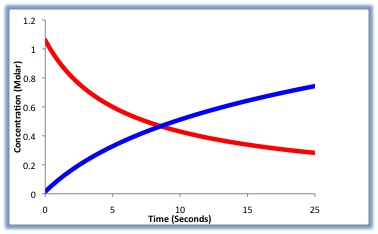
In the given hypothetical reaction: A+B⟶AB, the reactants A and B are consumed while the concentration of product AB increases. The reaction rate can be determined by measuring how fast the concentration of A or B decreases, or by how fast the concentration of AB increases. The graph below shows a reaction profile in which the reactants (red) decrease in concentration as the products increase in concentration (blue).
.jpg?revision=1&size=bestfit&width=378&height=234)
Answer the following questions based on the information above.
1. At what time is the reaction in equilibrium?
A. 6 s
B. 10 s
C. 8 s
D. 9 s
2. What is the concentration of the product at equilibrium?
A. 0.3 M
B. 0.4 M
C. 0.45 M
D. 0.5 M
3. During which time interval is the rate of the forward reaction greater than the rate of reverse reaction?
A. 0-9 s
B. 9-10 s
C. 10-15 s
D. 15-25 s
4. During which time interval is the rate of the reverse reaction greater than the rate of the forward reaction?
A. 0-9 s
B. 9-25 s
C. 5-9 s
D. 0-5 s
5. What is the concentration of the product at the beginning of the reaction?
A. 1.0 M
B. 0 M
C. 1.15 M
D. 0.5 M
6. What is the concentration of the reactants at the beginning of the reaction?
A. 1.0 M
B. 0 M
C. 1.15 M
D. 0.5 M
ANWSERS1. D 2. C 3. A 4. B 5. B 6. C
.jpg?revision=1&size=bestfit&width=378&height=234)